Radical Libre Ho2
A new method (ROxLIF) for the measurement of atmospheric peroxy radicals (HO2 and RO2) was developed using a two-step chemical conversion scheme and laser-induced.
Ribaucour et F. Louis, A theoretical study of the NCN ( 3Σ −) biradical thermochemical properties: Implications for combustion chemistry, Computational and Theoretical Chemistry, 2011, 967: 67-74 Voir aussi Bibliographie. S. Ribaucour et F. Louis, A theoretical study of the NCN ( 3Σ −) biradical thermochemical properties: Implications for combustion chemistry, Computational and Theoretical Chemistry. John D. (2015) Direct observation and kinetics of a hydroperoxyalkyl radical (QOOH); Science 6 February 2015:Vol.
643-646; DOI: 10.1126/science.aaa1495 Articles connexes.
Contents. Formation Hydroperoxyl is formed through the transfer of a hydrogen atom or even ion(s) to molecular oxygen, an to a or a proton to a. Reactivity The superoxide anion, O 2 −, and the hydroperoxyl radical are in in: O 2 − + H 2O ⇌ HO 2 + OH − The protonation/deprotonation equilibrium exhibits a of 4.88; consequently, about 0.3% of any superoxide present in the cytosol of a typical cell is in the protonated form. Unlike O 2 −, which predominantly acts as a reductant, HO 2 can act as an oxidant in a number of biologically important reactions, such as the abstraction of hydrogen atoms from and in the. As such, it may be an important initiator of. Because dielectric constant has a strong effect on p K a, and the dielectric constant of air is quite low, superoxide produced (photochemically) in the atmosphere is almost exclusively present as HO 2.
Definicion De Radical Libre

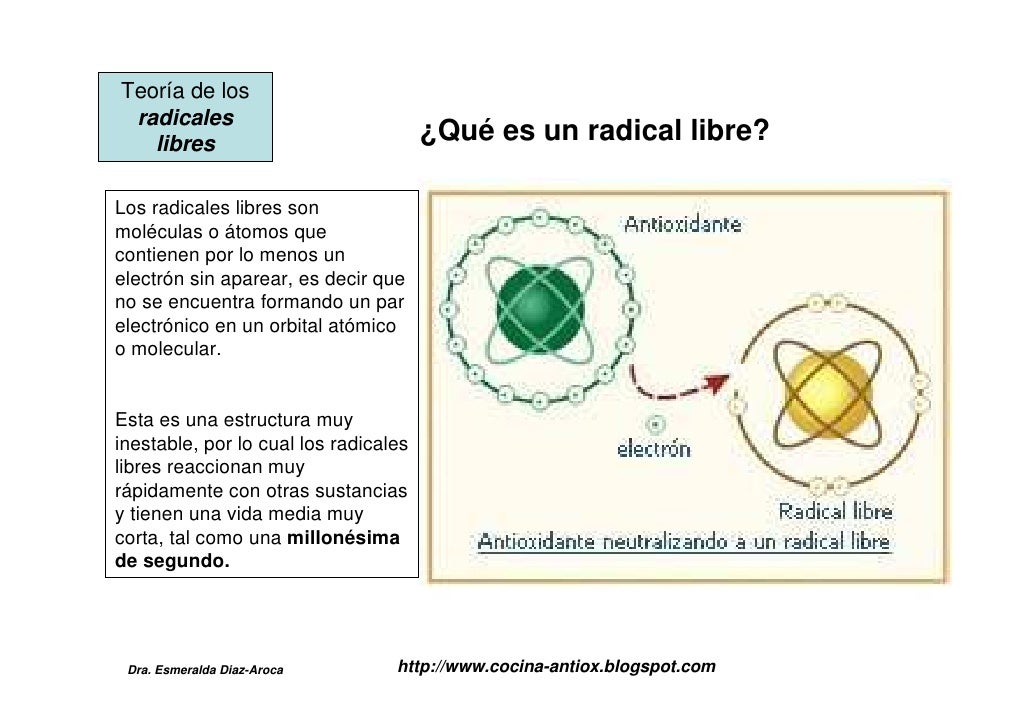
As HO 2 is quite reactive, it acts as a 'cleanser' of the atmosphere by degrading certain organic pollutants. As such, the chemistry of HO 2 is of considerable geochemical importance. Importance for atmospheric chemistry Gaseous hydroperoxyl is involved in reaction cycles that destroy stratospheric. It is also present in the troposphere, where it is essentially a byproduct of the oxidation of carbon monoxide and of by the radical. References.